Cellular Defence
Led by Silke Robatzek
Many microbes that colonize plants invade extracellular spaces. A physical barrier is thus present between the two organisms. Thus, the signal exchange between plants and microbes must occur across their cell surfaces. Pattern recognition receptors (PRRs) are primary sensors of the plant’s immune system, and presented at the plasma membrane to recognize pathogens and trigger immunity. Recent research has revealed the machinery that is essential for routing of PRRs and that endocytosis of PRRs is a mechanism for sustaining cellular responsiveness to confer long-term anti-bacterial immunity.
The FLAGELLIN SENSING 2 (FLS2) receptor for bacterial flagellin (flg22) is a prime example to investigate PRR trafficking. Plasma membrane-localized FLS2 is internalized in a clathrin-dependent manner and targeted towards distinct subcellular fates in dependence of its activation status. At the trans-Golgi network (TGN), non-activated FLS2 is recycled back to the plasma membrane. Activated (flg22-bound) FLS2 is ubiquitinated and sorted into multi-vesicular bodies (MVBs) of the late endosomal pathway destined for vacuolar degradation, which is regulated by the endosomal sorting complexes required for transport (ESCRT) machinery (See Figure 6a). In light of this, pathogen virulence effectors target TGN-localized trafficking regulators, and deregulation of the trafficking machinery i.e. mutants in clathrin or ESCRT components, enhances infection.
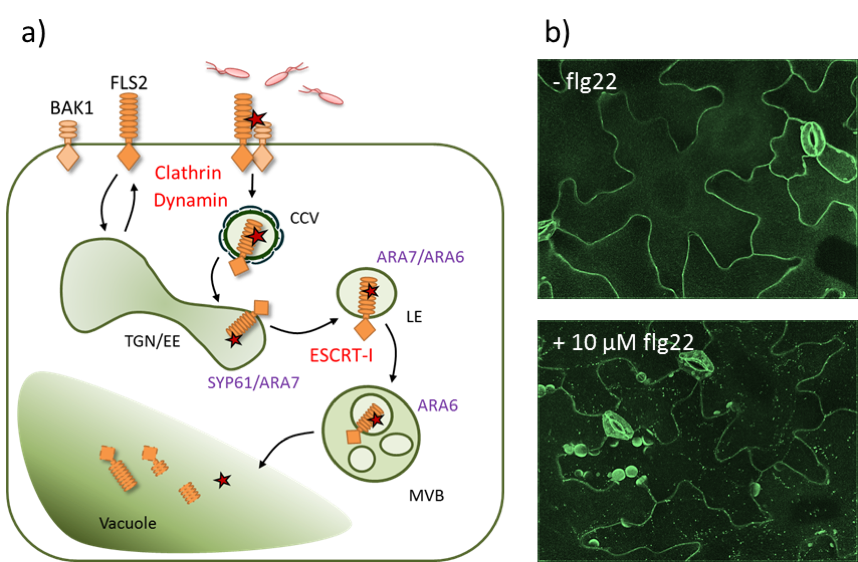
Figure 6: FLS2 endocytsosis. a) The PRR FLS2 and its co-receptor BAK1 localise at the plasma membrane. Upon flg22 perception, a receptor complex is formed and the activated receptor is internalised into clathrin-coated vesicles (CCV) and sorted into intraluminal vesicles of multi-vesicular bodies (MVBs) via the trans-Golgi-network (TGN)/early endosomes (EE) and the late endosomes (LE). Components required for FLS2 internalisation and sorting are indicated in red, endosomal marker proteins are indicated in lilac. b) Confocal micrographs (spinning disc microscopy) of FLS2-GFP before and after elicitation with flg22.
Following recognition of extracellular pathogens by PRRs, defence components must be delivered across the cell surface to inhibit pathogen growth. This involves significant reprogramming in secretory and endocytic trafficking, enabling focal accumulation at infection sites. Small Rab GTPases and syntaxins act at multiple steps of membrane trafficking, including cargo selection, vesicle formation, vesicle movement, tethering and membrane fusion. Distinct members of these families are associated with specific membrane compartments. Therefore, these trafficking regulators, i.e. the Rab5 GTPases ARA6 and ARA7 or the secretory ARA5 found at the MVB, TGN and Golgi, respectively, represent molecular probes not only to visualize focal accumulation and PRR trafficking along the endocytic route, but also to identify defence-related cargoes that are transported to pathogen infection sites.
Keynote Lecture
Paul Birch - The Delivery and Activity of late blight effector proteins that suppress plant immunity
James Hutton Institute, Dundee, UK
The oomycete Phytophthora infestans causes late blight, the most significant disease of potato and tomato. As such, it is a threat to food security. It has been shown to secrete so-called effector proteins to suppress the host immune system. Some of these effectors, notably the RXLR class, are delivered (or translocated) inside plant cells where they interact with host proteins, manipulating their function to promote disease. We have found that some of these RXLR effectors target and suppress the activity of plant proteins that are positive regulators of immunity. In contrast, other RXLRs target negative regulators of immunity, presumably supporting or promoting their activity to create a susceptible environment. This presentation will review both classes of RXLR effector activity, and where this occurs within the plant cell. In addition, we will look at how these effector proteins are translocated into the host cell from finger-like structures called haustoria that are formed by the pathogen during the early stages of infection.
About Paul Birch
After a PhD and post-doc in University of Manchester on the molecular biology of lignocellulose degradation by fungi, Paul Birch took up a Principal Investigator position at the Scottish Crop Research Institute (now James Hutton Institute) in Dundee in 1995. Since then he has studied the potato late blight pathogen, Phytophthora infestans, with a particular interest in the molecular and cellular processes governing the development of disease or disease resistance. His group has been instrumental in discovering effector proteins from the pathogen that enter plant cells and manipulate host defences. In 2007, he became ‘Professor of Plant Pathology’ at the University of Dundee. In 2016 he was made Fellow of the Royal Society of Edinburgh. His main interests are in understanding: • How pathogen effectors are delivered inside plant cells; • What host proteins and processes they target; • How they are recognised by host resistance proteins; and • How the complex co-evolution between host and pathogen dictates the outcomes of attempted infection.
Practical Session - Live Cell Imaging and Investigation of Subcellular Membrane Trafficking
Led by Gildas Bourdais, Michaela Kopischke, Agnieszka Siwoszek, Jelle Postma, Katarzyna Rybak, Janina Tamborski
Aims and Objectives
- Perform advanced fluorescence imaging using confocal laser scanning and spinning disc microscopy
- Generate images suitable for qualitative and quantitative analyses
- Provide a tool-box to understand and study the plant’s endomembrane trafficking machinery
In the practical sessions we will investigate the (co-)localisation of FLS2-GFP and various endosomal marker proteins following flg22 treatment using live-cell imaging approaches (See Figure 6 b). Chemical inhibitors and genetic interference with the endomembrane trafficking machinery will help us to dissect and understand the route of activated FLS2 in a temporal and spatial resolution.